Abstract
Background: Graft-versus-host disease (GVHD) is a dreaded complication after allogeneic stem-cell transplantation (ASCT). Previously, we and others showed that activation of type I interferon (IFN-I) inducing pathways such as RIG-I/MAVS or cGAS-STING can promote the integrity of the intestinal barrier and limit GVHD (Fischer et al., Sci Transl Med, 2017). However, the signals that drive these protective IFN-I responses are poorly understood.
Commensal microbiota can (i) have distant effects on immune responses through modulation of IFN-I signaling (Steed et al, Science, 2017; Swimm et al, Blood, 2018) and (ii) predict mortality in ASCT patients (Peled et al., N Engl J Med, 2020). We hypothesized that microbiota-derived products such as microbial metabolites engage IFN-I signaling in immune and non-immune cells poising them for induction of protective responses. We established a prospective, multi-centric clinical study in patients newly diagnosed with acute leukemia and performed longitudinal stool sampling to track changes in microbial community composition and metabolites expression levels. Submitted as a separate abstract to ASH 2021 (Orberg & Meedt et al.), we show that patients with high metabolite expression going into ASCT are less likely to develop GVHD. In this study, we translate our clinical observations to mouse models of acute GVHD and human and mouse intestinal organoids to uncover the molecular mechanisms via which metabolites protect the intestinal barrier during ASCT.
Methods: Stool samples from ASCT patients were obtained in Munich and at Regensburg in accordance to IRB-approved study protocols. Patients were sampled at initial diagnosis (Dx), prior to conditioning and weekly after ASCT up to day 28. We analyzed samples by 16S rRNA sequencing and mass spectrometry to obtain a complete picture of microbiome composition and function. Next, we tested metabolites which we detected in patients (desaminotyrosine [DAT], indole-3-carboxaldehyde [ICA]) as treatment in preclinical models in ASCT and GVHD mouse models. Outcomes were assessed by a novel organoid recovery assay in addition to established read-outs. To obtain a mechanistic understanding of the signaling pathways involved, we stimulated WT or IFN-I-signaling-impaired mouse (incl. STING -/-, MAVS -/-, IFNαR -/-) as well as human intestinal crypt-derived organoids with metabolites.
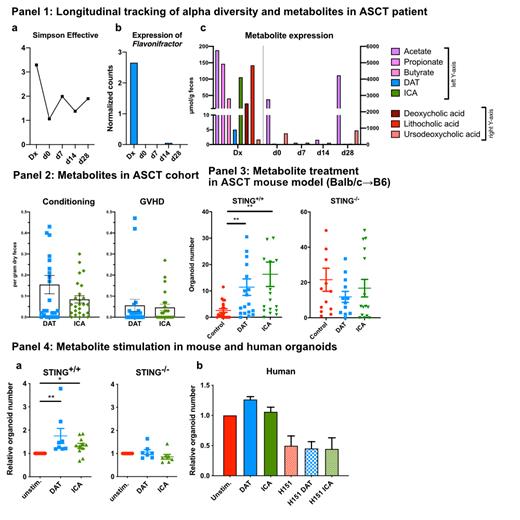
Results: Here, we present a 64-year-old female patient diagnosed with AML who received a 9/10 HLA-matched ASCT. At day 7, i.v. antibiotics were started due to fever and Enterococcus bacteraemia. At day 15, the patient developed skin and GI GVHD (Glucksberg III). At the timepoint initial diagnosis (Dx), i.e. the timepoint when we diagnosed AML but before therapy was initiated, we detected rich alpha diversity (Panel 1a) in the patient's stool. Flavonifractor plautii, a producer of the metabolite DAT, was detectable (Panel 1b). We observed high-level expression of metabolites including short-chain fatty acids, DAT, ICA and secondary bile acids (Panel 1c). Following ASCT, and especially at the early time-points day 0 and day 7, alpha diversity and metabolite expression declined drastically. We confirmed this trend in our multi-centric cohort of ASCT patients by comparing levels of DAT and ICA sampled at admission to the transplantation ward (Conditioning) versus at clinical diagnosis of GVHD: in patients with GVHD, metabolite levels were drastically reduced (Panel 2). Next, we prophylactically administered metabolites in a major mismatch mouse ASCT model. Metabolite-treated mice showed significantly showed better outcomes in our organoid recovery assay, which measures the ability of intestinal stem cells to recover after allogeneic injury (Panel 3). This effect that was abrogated in STING -/- recipients. Metabolite stimulation of mouse small intestinal organoids promoted organoid numbers and size, and required intact STING signaling (Panel 4a). Human colon organoids also responded to DAT and ICA, however the metabolite effect was lost when co-administered with the STING-inhibitor H151.
Conclusions: We identify that microbial-derived metabolites detected in patients can engage the STING pathway in humans and mice to confer resistance from immune damage. Thus, prophylactic administration of metabolite cocktails or bacterial consortia that can produce these metabolites may reduce occurrence of GVHD in ASCT patients.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal